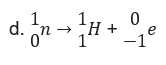12th class (Fsc) Physics unit 10 MCQs
26. The quantity of Ug35 in the naturally occurring uranium is:
a. 0.7%
b. 0.2%
c. 0.4%
d. 0.3%
27. Energy released by conversion of 1 amu is:
a. 200 MeV
b. 1.6×10-19eV
c. 931 MeV
d. 1.6×1019eV
28. Binding energy per nucleon is maximum for:
a. Uranium
b. Platinum
c. Lead
d. Iron
29. Nuclear fission chain reaction is controlled by using:
a. Platinum rods
b. Cadmium rods
c. Steel rods
d. Iron rods
30. The moderator used in a nuclear reactor:
a. Graphite
b. Sodium
c. Cadmium
d. Uranium
31. The charge on an alpha particle is equal to:
a. -2e
b. +e
c. 2e
d. -e
32. When a nucleus emits an alpha particle, its atomic mass decreases by:
a. 3
b. 1
c. 4
d. 2
33. Absorbed Does “D” is defined as:
a. C/m
b. m/E
c. E/m
d. E/C
34. 1 gray (Gy) is equal to:
a. 1Jkg
b. 1 Jkg-1
c. 1 Jkg-2
d. 1 kgJ-1
35. The reciprocal of decay construct λ of a radioactive element is:
a. Curie
b. Half life
c. Total life
d. Mean life
36. Which one of the following is not affected by electric or magnetic field?
a. X-rays
b. β-rays
c. γ-rays
d. Electron
37. For workers in nuclear facilities, a weekly does of-I normally considered safe:
a. 2.0 msv
b. 1.0 msv
c. 3.0 msv
d. 5.0 msv
38. There is no change in A and Z of any radioactive element by the emission of:
a. X-rays
b. α-rays
c. β-rays
d. γ-rays
39. The activity of radioactive sample:
a. Decreases linearly with time
b. Is constant
c. Decreases exponential with time
d. Increases with time
40. Curie is unit of:
a. Radioactivity
b. Conductivity
c. Resistivity
d. Binding energy
41. Gamma rays form cobalt-60 are used for treatment of:
a. Heart attack
b. Circulation of blood
c. Thyroid glands
d. Cancer
42. When r – rays are emitter the nuclear mass:
a. Increases by 2 units
b. Decreases by 4 units
c. Increases by 1 unit
d. Does not change
43. The element formed by radioactive decay is called:
a. Parent element
b. Father element
c. Daughter element
d. Mother element
44. Circulation of blood can be studied by using radioactive isotope:
a. Sodium-24
b. Cobalt-60
c. Iodine-131
d. Phosphours-32
45. The most useful tracer is:
a. Cobalt-60
b. Strontium-90
c. Carbon-14
d. Iodine-31
46. In Beta-decay reaction takes place:
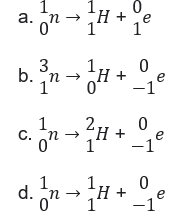
47. Marie Curie and Pierre Curie discovered:
a. Polonium and radium
b. Uranium
c. Radium
d. Uranium and radium
48. Thyroid cancer is cured by:
a. Cesium-131
b. Iodine-131
c. Carbon-14
d. Sodium-24
49. The most useful tracer isotope for the treatment of Thyroid gland is:
a. Iodin-131
b. Cobalt-60
c. Strontium-90
d. Carbon-14
50. Half life of radon gas is:
a. 3.8 months
b. 3.8 minutes
c. 3.8 year
d. 3.8 days