Chemistry Chapter 14 Entry Test MCQs
Topic 14
Nomenclature
1. The trivial name which was based upon the name of the area:
a. Marsh gas
b. Acetic acid
c. Urea
d. Barbituric acid
2. The common name of 2 – Methyl 2 chloropropane is
a. Ter. Butyl chloride
b. Iso. Buytl chloride
c. n – butyl chloride
d. Sec. butyl chloride
3. The IUPAC name of
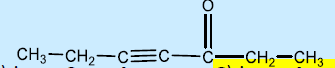
a. 3-oxo-2-heptyne
b. hept-3-yn-4-oxone
c. hept-4-yn-3-one
d. hept-3-yn-4-one
4. The incorrect IUPAC name is
a. (CH3)2CHCOCH2CH33 2-methyl-3-pentanone
b. CH3CH2CH2COOC2H5 ethylbutanoate
c. (CH3)2CHCH(OH)CH3 2-methyl-3-butanol
d. (CH3)2CHCH2CHO 3-methyl butanal
5. The IUPAC name of CH3 – CH = CH – C ≡ CH is
a. Pent-3-en-4-yne
b. Pent -3-en-1-yne
c. Pent-2-en-4-yne
d. Pent-2-en-3-yne
6. Which of the following matches of names and molecules are correct?
a. Ketone III. CH3OCH3
b. Alcohol I. HCOOH
c. Acid IV. CH3COCH3
d. Aldehyde II. (CH3)3COH
7. Select the IUPAC name for: (CH3)2CHCH(OH)CH2C(CH3)3.
a. 1,1,4,4-pentamethylbutanol
b. 2,5,5-trimethyl-3-hexanol
c. 2,5-dimethyl-4-hexanol
d. 1,1-dimethylisopentanol
8. Which compound is different from the others?
a. methyl propyl ketone
b. methyl ethyl ketone
c. 2-pentanone
d. pentan-3-one
9. Which is the correct IUPAC name of the following compound?
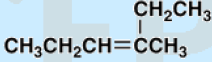
a. 4-ethylpent-3-ene
b. -ethylpent-2-ene
c. 3-methylhex-3-ene
d. 4-methylhex-3-ene
10. Select the correct IUPAC name for:
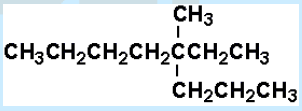
a. 5-methyl-5-ethyloctane
b. 5-methyl-5-propylheptane
c. 4-ethyl-4-methyloctane
d. 3-methyl-3-propyloctane
11. Select the best name for:
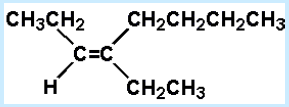
a. 4-ethyl-cis-3-octene
b. 4-ethyl-trans-3-octene
c. 4-butyl-cis-3-hexene
d. 5-ethyl-trans-5-octene
12. Name of the compound given below is
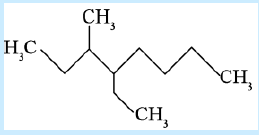
a. 3-methyl-4-ethyloctane
b. 4-ethyl-3-methyloctane
c. 2, 3-diethylheptane
d. 5-ethvl-6-methvloctane
13. The IUPAC name of CH3CH2COCl is
a. Acetyl chloride
b. Propanoyl chloride
c. Chloroethane
d. Ethanoyl chloride
14. How many methyl group are present in 2,5-dimethyl-4-ethylheptane?
a. 4
b. 2
c. 5
d. 3
15. The IUPAC name of the following compound CH3 – C (CH3)2 – CH = C(CH3)2 is
a. 1, 3, 3-Trimethyl-pent-2-ene
b. 1, 1, 3, 3-Tetramethyl-but-1-ene
c. 2, 4, 4-Trimethylpent-2-ene
d. 2, 2, 4-Trimethylbut-4-ene
16. IUPAC name of CH3 CH = C (OH) – CH3 is
a. 2-butene-2-ol
b. butylene alcohol
c. bute 2-ene 1-ol
d. 2-butene-3-ol
17. The IUPAC name of the following compound is (CH3)2C= CH- CH2 – C(CH3)3
a. 2,4-Dimethyl heptene
b. 2,2,5-Trimethyl- 4-hexene
c. 2,5-Dimethyl heptene
d. 2,5,5-Trimethyl-2-hexene
Fission
18. The central C – atom of free radical possesses:
a. 7 electrons
b. 6 electrons
c. 4 electrons
d. 8 electrons
19. Methyl free radical involve:
a. Sp2 hybridisation
b. Sp hybridization
c. sp3d hybridization
d. Sp3 hybridisation
20. Tertiary butyl carbocation involves:
a. Sp2 hybridisation
b. Sp hybridization
c. sp3d hybridization
d. Sp3 hybridisation
21. With a change in hybridisation of the carbon bearing the charge, the stability of a carbanion increase in the order
a. sp3 < sp2 < sp
b. sp < sp2 < sp3
c. sp2 < sp < sp3
d. sp < sp3 < sp2
22. Which of the following require an electron to complete its octet?
a. :CCl2
b. Cl+
c. NO2+
d. Cl•
23. The heterolytic fission of C – Br bond in case of CH3CH2Br result into the formation of:
a. Carbanion
b. Carbene
c. Allyl radical
d. Carbonium ion
24. The order of stability of carbonium ions is
a. Tert-butyl > iso-propyl > ethyl > methyl
b. Methyl > ethyl > iso-propyl > tert-butyl
c. Tert-butyl > ethyl > iso-propyl > methyl
d. Iso-propyl > tert-butyl > ethyl > methyl
25. Incorrect statement about free radicals is
a. Hybridization of carbon containing unpaired electron is always sp2
b. They have same stability order as carbocation’s
c. They are paramagnetic
d. All are correct
