Chemistry Chapter 14 Entry Test MCQs
76. The ozonolysis of 1-butene gives:
a. C2H5CHO, HCHO
b. CH3CHO, HCHO
c. CH3CHO, HCHO
d. CH3CHO, CO2
77. Raney Nickel is prepared by reacting an alloy with caustic soda:
a. Ni-Al
b. Ni-Cu
c. Ni-Mg
d. Ni-Fe
78. In the following sequence of reactions, the product is
![]()
a. Propene
b. Propan-1-ol
c. Propyne
d. Propan-2-ol
79. Identify B and D in the following sequence of reactions.
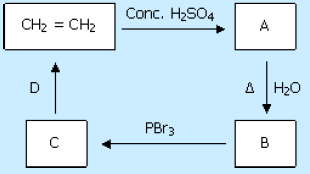
a. Ethanol and alcoholic KOH
b. Methanol and bromoethane
c. Ethyl hydrogen sulphate and alcoholic KOH
d. Ethyl hydrogen sulphate and aqueous KOH
Alkynes
80. When 2-butyne is treated with dil. H2SO4/HgSO4, the product formed is
a. 1-Butanol
b. 2-Butanol
c. Butanone
d. Acetone
81. The end product in the sequence of reactions is
![]()
a. Ethanol
b. acetone
c. acetic acid
d. isopropyl alcohol
82. Reaction of acetylene and propylene with HgSO4 in presence of H2SO4 produces respectively
a. acetaldehyde and acetone
b. acetone and acetaldehyde
c. acetone and propanaldehyde
d. propanaldehyde and acetone
83. Acrylonitrile is formed when acetylene is treated with:
a. HCN in presence of Cu2Cl2/NH4Cl
b. HCN in presence of Cu2Cl2/NH4OH
c. HCN in presence of CuCl2/NH4OH
d. HCN in presence of CuCl2/NH4Cl
84. Alkynes when burnt in air to produce CO2 and H2O, which is used for welding and cutting of metals.
a. Oxyacetylene flame
b. Acetylene flame
c. steam flame
d. CO2 flame
85. Sodium acetylide is very valuable reagent for organic synthesis and nature is
a. Covalent
b. Co-ordinate
c. Non-polar
d. Ionic
86. Acetylene reacts with ammonical AgNO3 as follow
HC=CH+AgNO3+NH4OH→AgC=CAg+NH4NO3+H2O The mechanism of above reaction is
a. electrophilic substitution
b. free radical substitution
c. redox reaction
d. nucleophilic substitution
87. The compound which reacts with HBr obeying Markownikov’s rule is
a. 2-methyl-2-butene
b. Ethene
c. trans-2-Butene
d. cis-2-Butene
88. When lime is heated with coke at 2800˚C in an electric furnace, the products formed are
a. CaC2 + CO
b. CaO+CO
c. CO+CO2
d. CaC2+CO2
89. Ammonical silver nitrate gives white precipitates with
a. C6H6
b. C2H4
c. C2H2
d. C2H6
90. Alkynes can be converted into trans-alkenes by using:
a. Zn/Hg + HCl
b. N2H4/KOH
c. Pd, BaSO4/Quinoline
d. Na/NH3(liquiD) , -33.4°C
91. Acetylene and ethylene reacts with alkaline KMnO4 to give:
a. ethyl alcohol and ethylene glycol
b. Oxalic acid and a formic acid
c. Oxalic acid and ethylene glycol
d. Acetic acid and ethylene glycol
92. Acetylene is polymerized into divinylacetylene and benzene. What property is same in the products?
a. Atomic ratio
b. Hybridization
c. Atomic nature
d. Reactivity
Benzene
93. The carbon-carbon and C-H bond lengths in benzene molecule are respectively
a. 133 pm, 108 pm
b. 154 pm, 108 pm
c. 1.25 A°, 1.08 A°
d. 139.7 pm, 109 pm
94. Which of the following is not observed in the combustion of pure methane in a plenty of air?
a. The flame is smoky
b. Water is produced
c. Energy is released
d. CO2 is produced
95. Toluene can be converted into benzoic acid on reacting with:
a. Conc. HNO3
b. dil. NaOH
c. Acidified KMnO4
d. dil. HNO3
96. Under which of the following toluene shows side chain substitution reaction:
a. Cl2 in presence of AlCl3
b. Cl2 in presence of UV light
c. Hydrogen in presence of FeCl3
d. CH3COCl in presence of AlCl3
97. The ratio of sigma to pi bonds in benzene is :
a. 4
b. 2
c. 8
d. 3
98. The correct decreasing order of priority for the functional groups of organic compounds in the IUPAC system of nomenclature is
a. –SO3H, – COOH – CONH2, – CHO
b. –COOH, – SO3H, – CONH2, – CHO
c. –CONH2, – CHO, – SO3H, – COOH
d. –CHO, – COOH, – SO3H, – CONH2
99. The function of anhydrous AlCl3 in the Friedel-Craft’s reaction is to
a. Absorb HCl
b. To produce electrophile
c. Absorb water
d. To produce nucleophile
100. Anhydrous AlCl3 is used in the Friedel-Craft’s reaction because it is
a. Soluble in ether
b. Electron rich
c. Electron deficient
d. Insoluble to chloride ions
