Physics Chapter 12 Entry test MCQs
Topic 12
Atomic Spectra
1. When a hydrogen atom is in its first excited level, what is the relation of radius and Bohr radius?
a. Same
b. Twice
c. Half
d. 4 times
2. What is the energy required to ionize an H-atom from the third excited state, if ground state ionization energy of H-atom is 13.6 eV?
a. 13.6 eV
b. 0.85 eV
c. 12.1 eV
d. 3.4 eV
3. 1/λr = R (1/P2 -1/n2) in this relation P = 1, 2, 3 and n will be
a. P, P-1, P-2….
b. P, P+1, P+2,……..
c. P-1, P-2….
d. P+1, P+2….
4. Lyman series of H-atoms lie in the———-region of electromagnetic spectrum
a. infrared
b. visible
c. red
d. ultraviolet
5. The Balmer series for hydrogen atom corresponds to electronic transitions that terminate in the state of quantum number n = 2 The longest wavelength of photon emitted is
a. 36/5R
b. 5R/36
c. 5/36
d. 36/5
6. The hydrogen atoms are excited to the stationary state designated by the principal quantum number n = 4 The number of maximum spectral lines are observing
a. 3
b. 2
c. 6
d. 4
7. Bracket series is obtained when all the transition of electron terminate at
a. Second orbit
b. First orbit
c. Fourth orbit
d. Third orbit
8. Number of the emission spectra are
a. Two
b. One
c. Four
d. Three
9. When electron jumps from nth to the pth orbit in an hydrogen atom then the wavelength of the emitted radiation is given by
a. 1/λ = RH [1/n2 – 1/p2 ]
b. 1/λ = RH [1/p2 – 1/n2 ]
c. 1/λ = 1/RH [1/42 – 1/n2]
d. 1/λ = 1/RH [1/p2 – 1/n2 ]
10. Photons of highest frequency emitted in Lyman series is given as:
a. f =RHc
b. f = c/RH
c. f = 1/RHc
d. f = RHc
11. Which of the following is true for number of spectral lines in going from Lyman series to P-fund series?
a. Unchanged
b. Increases
c. May decreases or increases
d. Decreases
12. Number of spectral lines in hydrogen atom is
a. 15
b. 3
c. infinite
d. 6
13. Figure shows the energy levels P,Q,R,S and G of an atom where G is the Ground state. A red line in the emission spectra of the atom can be obtained by an energy level change from Q to S. A blue line can be obtained by following energy level change
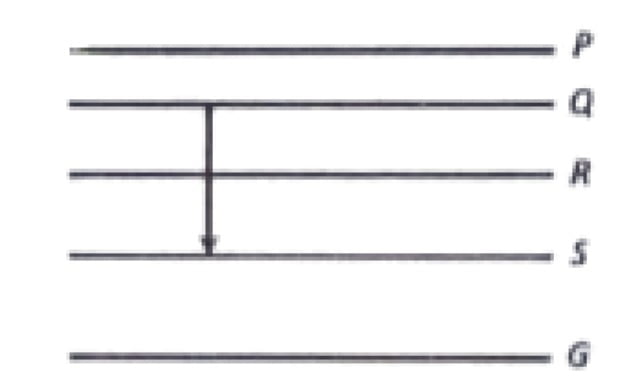
a. R to S
b. Q to R
c. R to G
d. P to Q
14. The maximum wavelength of Lyman series is……
a. C/R
b. 4/3R
c. 1/RC
d. 1/R2
15. An atom is excited to an energy level E1 wavelength of the radiation emitted is from its ground state energy level Eo. The
a. (E1 – E0) / h
b. (Eo – E1) / hc
c. hc/(E1 – E0)
d. E1/hc – E0/hc
16. Which of the transitions in hydrogen atom emits a photon of lowest frequency ( n = quantum number)
a. n = 3 n = 1
b. n = 2 n = 1
c. n = 4 n = 2
d. n = 4 n = 3
17. During the transition of Electron of Hydrogen atom from higher orbit to a third orbit, a photon of:
a. Balmer series is emitted
b. Paschen series is emitted
c. Brakett series is emitted
d. Lyman series is emitted
18. The radiations emitted from hydrogen filled discharge tube shows:
a. Line spectrum
b. Continuous spectrum
c. Band spectrum
d. None of these
19. An electron jumps 4th orbit to the 2nd orbit of hydrogen atom. Given the Rydberg’s constant R = 105cm – 1 The frequency in Hz of the emitted radiation will be
a. 9/16×1015
b. 3/16 x 105
c. 3/4 x 1015
d. 3/16 x 1015
20. If an electron jumps from 1st orbital to 3rd orbital, then it will.
a. No gain of energy
b. Absorb energy
c. Release energy
d. None of these
21. The ratio of the frequencies of the long wavelength limits of Lyman and Balmer series of hydrogen spectrum is
a. 4:1
b. 27:5
c. 1:4
d. 5:27
22. The electron in hydrogen atom makes a transition from n = 2 energy state to the ground state n = 1 The wavelength of emitted photon is
a. 4/3R
b. 3R/4
c. 4/3
d. 3/4
23. Shortest wavelength photon in the Balmer series is
a. 1/4
b. 4/R
c. 4
d. R/4
24. To find longest wavelength radiation in Balmer series, the value of n used in
a. 3
b. 2
c. Infinity
d. 4
25. Photon of highest frequency will be absorbed when transition takes place from:
a. 3rd to 5th orbit
b. 1st to 5th orbit
c. 4th to 5th orbit
d. 5th to 1st orbit
26. Balmer series lies in that region of electromagnetic wave spectrum, which is known as:
a. Invisible region
b. Visible region
c. Infra-red region
d. Ultraviolet region
27. The relation for Paschen series is given as
a. 1/λ = RH (1/32 – 1/n2)
b. 1/λ = RH (1/22 – 1/n2)
c. 1/λ = RH (1/52 – 1/n2)
d. 1/λ = RH (1/42 – 1/n2)
Past papers mcqs
28. Which of the following series lies in ultraviolet region?
a. Lyman series
b. Balmer series
c. Bracket series
d. Pascher series
