Physics Chapter 13 Entry test MCQs
Topic 13 (Nuclear Physics )
PRACTICE EXERCISE
1. What is the half-time of a radioactive sample (in minutes), if its mean life is 200s?
a. 2.57 min
b. 0.69 min
c. 2.31 min
d. 2 min
2. What will happen in a time of 7 hours, if a radioactive substance has an average life of 7 hours?
a. More than half of the active nuclei decay
b. Half of the active nuclei decay
c. All active nuclei decay
d. Less than half of the active nuclei decay
3. The difference between U235 and U238 atom is that
a. U238contains 3 more protons and 3 more electrons.
b. U238 contains 3 more protons
c. U238 contains 3 more neutrons
d. U238 contains 3 more neutrons and 3 more electrons.
4. Which two nuclei contain the same number of neutrons?

5. What is the ratio of the nuclear densities of two nuclei having mass numbers in the ratio 1:4?
a. 1:4
b. 1:1
c. 1:3
d. 1:2
6. The radius for an atom is —times the radius of the nucleus.
a. 1015
b. 105
c. 1020
d. 1010
7. a, ẞ and y are emitted from a radioactive substance
a. When it interacts with the other particles
b. Spontaneously
c. When it is exposed to light
d. When it is heated
8. The order of penetration power of a and ẞ and y-rays is
a. a > ẞ < r
b. a > ẞ > r
c. a < ẞ < r
d. a < ẞ > r
9. in a given reaction 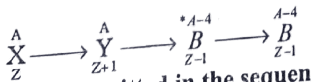
radioactive radiations are emitted in the sequence
a. γ, β, γ
b. β, α, γ
c. γ, α, β
d. α, γ, β
10. When boron 10/5 B is bombarded by neutrons, a-particles are emitted. The resulting nucleus has the mass number
a. 7
b. 11
c. 15
d. 6
11. In which radioactive disintegration neutron dissociates into proton and electron?
a. y-emission
b. B-emission
c. a-emission
d. None of these
12. 92U238 nucleus emits two a-particles and two B-particles and transforms into a thorium nucleus. Which of the following is the mass number and atomic number of the thorium nucleus so produced?
a. 234, 90
b. 230, 90
c. 234, 88
d. 230, 88
13. Specify the parent, daughter nuclei emitted particle in the given reaction
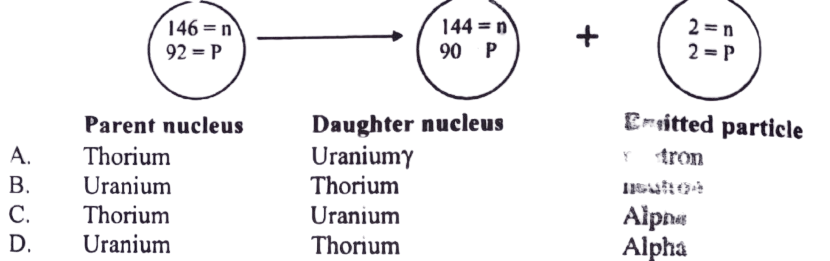
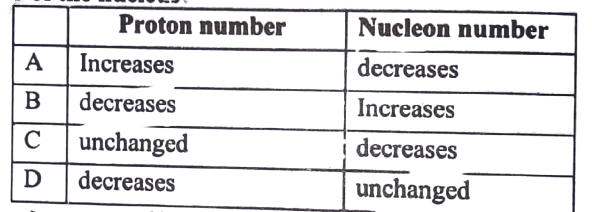
14. The Decay of nucleus is accompanied by the emission of two Beta particles and Alpha Radiations. what effect (if any) does this have on the proton number and the nucleon number of the nucleus?
15. a-particle is bombarded on 14N as a result 17O is formed. The particle emitted is
a. Proton
b. Neutron
c. Positron
d. Electron
16. If 92U233 decays twice by a-emission, the resulting isotopes will be
a. 225Rn86
b. 225Ra88
c. 229Th90
d. 234pa88
17. The following represents a sequence of radioactive decays involving two Alpha particles and one Beta Particles ![]()
what is the nuclide X?
a. 213At85
b. 209Pb82
c. 215Ir77
d. 217 TI 81
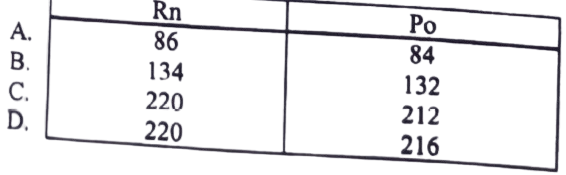
18. Radon-220 is radioactive and decays to polonium-216 with the emission of an Alpha particles. The equation for the radio active decay is shown ![]() how many neutrons are in the radon and polonium nuclei?
how many neutrons are in the radon and polonium nuclei?
19. In alpha decay, the ratio of decrease in proton number to the decrease in neutron number is
a. 1:2
b. 2:1
c. 4:1
d. 1:1
20. Radon 222 Rn 86 decays by Alpha and Beta Emission to bismuth 214 Bi 83 for the decay of each nucleus of radon. how many alpha and beta particles are emitted?
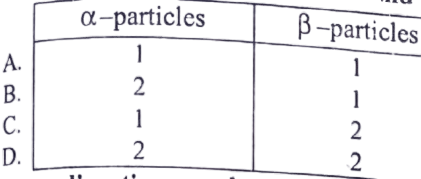
21. A radioactive nucleus undergoes a series of decay according to the scheme ![]() if the mass number and the atomic number of A are 180 and 72 respectively, then what are these numbers for A4
if the mass number and the atomic number of A are 180 and 72 respectively, then what are these numbers for A4
a. 172 and 69
b. 176 and 69
c. 174 and 70
d. 176 and 70
22. neutron decay in the free space is given as follows ![]() then the parenthesis represents an
then the parenthesis represents an
a. photon
b. neutrino
c. Graviton
d. Antineutrino
23. The ratio of the rate of decay of a parent atom to the number of radioactive nuclei present at that time is equal to:
a. Decay constant
b. Half-life
c. Activity
d. Mean life
24. The masses of two radioactive substances are same and their half-lives are 1 year and 2 years respectively. The ratio of their activities after six years will be:
a. 1:8
b. 1:4
c. 8:1
d. 4:1
25. Let T be the mean life of a radioactive sample. 75% of the active nuclei present in the sample initially will decay in time.
a. 1/2 ( ln 2 )T
b. 2T
c. 2 ( ln 2 )T
d. 4T
