Physics Chapter 13 Entry test MCQs
51. Decay constant ‘λ’ is given a

52. Ionizing capability of gamma rays is:
a. Less than alpha but greater than beta particles
b. Equal to alpha and beta particle
c. Less than beta but greater than alpha particles
d. Less than both alpha and beta particles
53. Half-life of a radioactive element is:
a. Half-life of a radioactive element is:
b. Inversely proportional to square of decay constant
c. Inversely proportional to decay constant
d. Directly proportional to decay constant
54. The transformation of a neutron into proton in the nucleus gives rise to emission of:
a. Alpha particles
b. Beta particles
c. X-rays
d. Gamma particles
55. The ratio of the rate of decay of a parent atom to the number of radioactive nuclei present at that time is equal to:
a. Mean life
b. Half-life of radioactive element
c. Activity if radioactive element
d. Decay constant of radioactive element
56. What is the charge on alpha particles emitted during the phenomenon of radioactivity?
a. -e
b. +e
c. +2e
d. -2e
57. A radioactive nuclide decays by emitting an x-particle and a y-ray photon, the change in the nucleon number will be:
a. -2
b. -4
c. -3
d. -2
58. A half-life of sodium-24 is———–which is used to estimate the volume of blood in a patient:
a. 15 hours
b. 15 hours
c. 15 days
d. 8 hours
59. In a radioactive phenomenon, observation shown in figure where a deviates lesser than ẞ in same electric or magnetic field (not shown in the figure). What is the reason of less deviation of α?
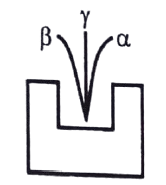
a. α is lighter particle
b. α is heavier particle
c. α is very fasting moving particle
d. none of these
60. Which of the following effect is observed due to emission of ẞ during the phenomenon of radioactivity?
a. Z increases by 1 and A remains same
b. A increases by 1 and Z remains same
c. A decreases by 1 and Z remains same
d. Z decreases by 1 and A remains same
61. Isotopes are those nuclei of an element that have:
a. Same mass number as well as atomic number
b. Same mass number as well as atomic number
c. Same atomic number but different mass number
d. Different mass number as well as atomic number
62. Emission of alpha decay from a radioactive substance cause:
a. Decreases in ‘A’ by 1 and ‘Z’ remains same
b. Decrease in ‘Z’ by 4 and decrease in ‘A’ by
c. Decrease in ‘A’ by 4 and decrease in ‘Z’ by 2
d. Decrease in ‘Z’ by 1 and ‘A” remains same
63. Which one of the following emissions takes place in a nuclear reaction? 90Th234 → 91Pa234 + ————
a. Gamma
b. Alpha
c. Photons
d. Beta
64. Among the three types of radioactive radiation, which have strongest penetration power?
a. Gamma
b. Alpha
c. a, ẞ and y have same penetration
d. Beta
65. Emission of radiation from radioactive substance is
a. Independent of temperature but dependent on pressure
b. Dependent on both temperature and pressure
c. Independent of pressure but dependent on temperature
d. Independent of both temperature and pressure
66. In the nuclear reaction 11Na24 → 12Mg24 + X .The particle X is?
a. Electron
b. Positron
c. Proton
d. Neutron
67. Three points of radioactive radiation are observed as shown in the figure presence of electric field, which type of radiation is shown in the path ‘1’?
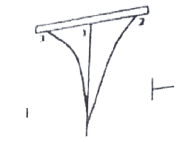
a. Alpha
b. Beta
c. Gamma
d. Cathode Rays
68. A Beta Particle is fast moving electron, during a Beta Decay how the atomic number and mass number of a nucleus change?

69. A uranium isotope 92U234 undergoes one a-decay and one o -1B-decay. What is the atomic number of the final product?
a. 91
b. 90
c. 88
d. 89
70. A naturally occurring radioactive element decays two alpha particles. Which one of the following represents the status of daughter element with respect to mass number A and charge number 2?
a. Z decreases by 2 and A decreases by 4
b. Z decreases by 4 and A decreases by 2
c. Z decreases by 8 and A decreases by 4
d. Z decreases by 4 and A decreases by 8
71. A radioactive isotope W decay to x which decay to Y and Y decays to Z as represented by the figure below.
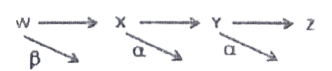
What is the change in the atomic number from W to Z?
a. increases by 3
b. increases by 5 c. decreases by 3
d. decreases by 5
72. In the reaction 90Th234 → 91Pa234 + -1e0 the electron -1e0 emits from the
a. 1st Orbit
b. Nucleus
c. 2nd Orbit
d. Valence Shell
73. According to the equation Azx ——>Y + 3a-particles. what are the atomic and mass numbers of ‘Y’?
a. Z-2, A-4
b. Z-6, A-12
c. z + 3 A
d. Z+1, A
74. A certain radioactive nuclide of mass number ‘x’ decay by ẞ-emission and a-emission to a second nuclide of mass number ‘t’, which of the following correctly relates ‘x’ and ‘t’?
a. x – 1 = t
b. x = t – 4
c. x = t + 4
d. x + 3 = t
75. During the decay of radioactive isotopes overline m infty X to a stable isotope, six a-particles and four ẞ-particles are emitted, what is the atomic number ‘Z’ and mass number ‘A’ of the stable isotopes:
a. Z = 78 A = 212
b. Z = 70 A = 220
c. Z = 82 A = 208
d. Z = 82 A = 212
